Here at StudyOrgo, we frequently get questions about topics in organic chemistry that are usually quickly covered, poorly described or expected that you know from previous courses. These concepts are really important to understanding the more complex topics to come. In this article, we will cover the concepts of stereochemistry descriptions using bold and wedged bonds. This is just a preview of the detailed topics and materials available with your membership to StudyOrgo.com. Sign up today!
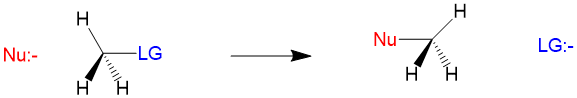
Substitution reactions involve the attack by an electron-rich element, referred to as the nucleophile, on an electron-poor atom, referred to as the electrophile. As the reaction name suggests, we are substituting the nucleophile for another group on the electrophile atom, which is referred to as the leaving group. The generic reaction looks like this.
In Substitution reactions, there are two mechanisms that will be observed. An Sn2 and Sn1 reaction mechanism.
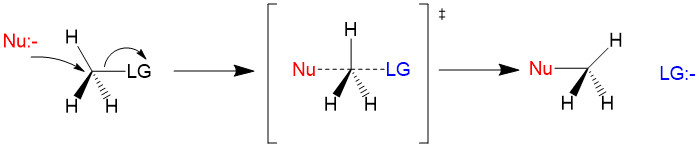
Sn2 reactions are bimolecular in rate of reaction and have a concerted mechanism. The process involves simultaneous bond formation by the nucleophile and bond cleavage by the leaving group. The transition state looks like this. Because the reaction is concerted, Sn2 mechanisms will always lead to an inversion of stereochemistry! For reactivity using an Sn2 mechanism, primary >> secondary >> tertiary carbon centers.
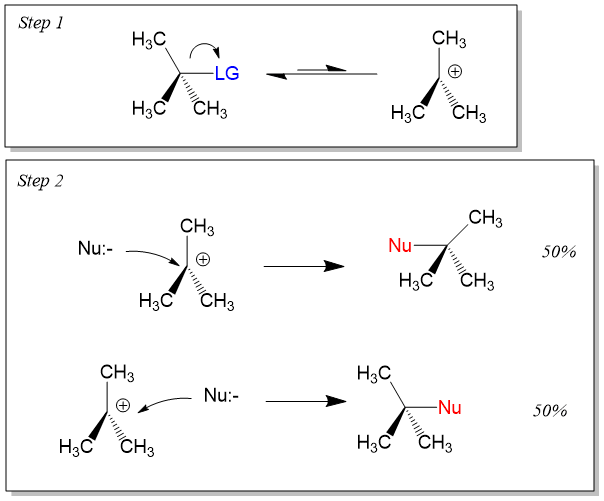
On the other hand, Sn1 reactions are unimolecular in rate of reaction and have a step-wise mechanism. This process first involves bond cleavage by the LG to generate a carbocation intermediate. The stability of carbocation formation will determine if Sn1 or Sn2 reactions occur. In the second step, the electronegative nucleophile attacks the carbocation to form the product. The steps look like this. Because the nucleophile can attach either side of the carbocation, which adopts an sp2-hybridized orbital with a trigonal planar geometry, an equal amount of inversion and retention is seen, referred to as a racemic mixture. For reactivity using an Sn1 mechanism, tertiary >> secondary >>> primary carbon centers.
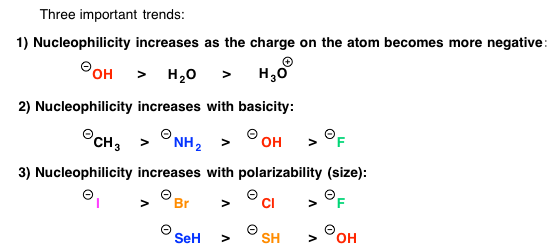
The strength of nucleophiles used help to determine the reaction mechanism. Strong bases will almost always proceed to Sn2 mechanism. Weak nucleophiles will generally proceed to Sn1 mechanism when a stable carbocation is present. Below is a list of nucleophile trends in order of nucleophile strength.
We hope that this learning aid will help you answer any questions you may have had about Sn2 and Sn1 reactions. We here at StudyOrgo have compiled hundreds of reactions with clear explanations to help you speed up your studying and get a great grade in organic chemistry. Sign up today to get access to all of our reactions!