Q&A from our students:
Question: What are primary, secondary and tertiary carbons? I know that sounds like a basic questions, but were just beginning to learn about alkanes and stuff and I don’t get it. Thanks.
Answer: It is a great questions and we’re am happy you asked because there are a lot of students who are confused about this subject. Let’s try to clear the air. Because here at StudyOrgo.com – we love to clear the air and make things easy!
We use the terms primary, secondary, and tertiary to refer to the substitution level that a given carbon has in a molecule. In other words, these terms are used to describe how many other carbons a given carbon is attached to.
So to figure out the substitution level of any given carbon, follow these three easy steps:
Step #1: Pick a carbon
Step #2: Count how many carbons are directly attached to it. Other elements such as hydrogen, nitrogen, oxygen etc. don’t count.
Step #3: Give it a label:
- Primary = a carbon attached to only ONE other carbon
- Secondary = a carbon attached to only TWO other carbons
- Tertiary = a carbon attached to THREE other carbons
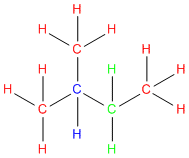
In the below example each carbon is color coded using the labels in step #3 above.
Go ahead give it a try!
OK- now bear in mind that hydrogens attached to a given carbon ALSO take on the labels as described in step #3 above.
So we can apply the same principle to the hydrogens:
- Primary = a hydrogen on a carbon attached to only ONE other carbon
- Secondary = a hydrogen on a carbon attached to only TWO other carbons
- Tertiary = a hydrogen on a carbon attached to THREE other carbons
For more on mastering alkanes and reactions, use coupon code “acespring” to save 10% off the highest pass rate organic chemistry program.
You may submit a question to our experts by filling out the form HERE.
You’re question may be answered in an upcoming blog posting!