The start of first semester organic chemistry can be an information overload. For the first few classes, you will review general chemistry concepts and then… the reactions start coming! One of the first reactions that will be covered is the SN2 reaction, mainly because it is relatively straight forward and a good tutorial for how to describe reaction mechanisms. In this article, we will review the important topics of an SN2 reaction. Sign up with StudyOrgo today to get detailed reaction mechanisms and explanations to stay on top of your class!
Alkyl halides as SN2 substrates
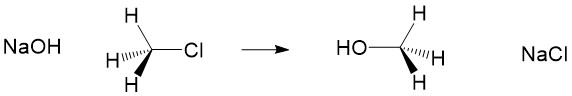
One of the most reactive molecules involving substitution reactions are alkyl halides. However, there are a number of considerations to keep in mind to determine if the SN2 mechanism describes your reaction. First, let’s look at a simple SN2 reaction; methyl chloride and NaOH to form methanol and NaCl.
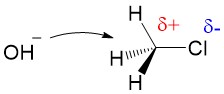
Let’s break down the reaction mechanism into the basic elements. An SN2 reaction gives you 3 pieces of information, first the ‘S’ indicating ‘substitution’, the ‘N’ denoting the reaction involves a nucleophile and ‘2’ describing the process as bimolecular – meaning both the substrate and the nucleophile determine the rate of the reaction. The hydroxide will attack the carbon center and form a new bond with carbon (which makes it the nucleophile) and the chlorine atom will leave the carbon center with the electrons from the C-Cl bond (which makes it the leaving group).
Inductive effects of leaving groups: Chloride is a good leaving group because of the inductive effects (or electron withdrawing potential) of the halogen atom. This is the characteristic of good leaving groups. The electronegativity of chlorine makes the carbon center slightly electrophilic, meaning it has a partial positive charge, which is strongly attracted to electron-rich nucleophiles.
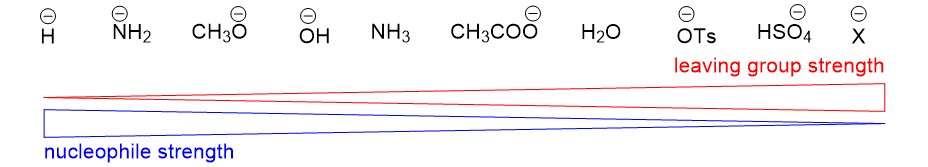
Strong bases as a nucleophile: In order to form a new bond with carbon, a good nucleophile has to be electron rich. The strong basic properties of NaOH make the charge on oxygen negative, and thus a good nucleophile. Likewise, the poor basic properties of Cl anion make it an excellent leaving group. Below is a chart to help illustrate the contrasting properties of nucleophiles and leaving groups.
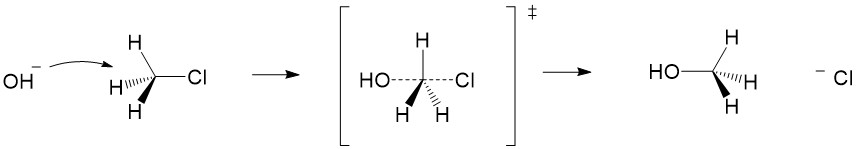
Inversion of stereochemistry due to geometry of attack: Once the nucleophile attacks the carbon center, a partial formation of C-O bond and breaking of C-Cl bond occurs in a concerted (or instantaneous) fashion, depicted below. Because the angle of attack for the nucleophile has to be opposite of the leaving group, the OH adds to the opposite side of the carbon center, causing an inversion of stereochemistry. This is an important clue in determining if reactions occur using the SN2 mechanism.