Many of our students are unclear on a number of key terms that will be necessary to move forward in organic chemistry. We have compiled a list of commonly misunderstood terms and explain them here. With this review, our quiz mode review of all of the reactions you have learned and descriptions from StudyOrgo.com, you will be sure to boost your final exam score and get a great grade in your class!
Isomers – There are two types of isomers in organic chemistry.
- Constitutional isomers – two or more molecules with the same number of atoms but in a different geometrical arrangement (i.e. different connectivity).
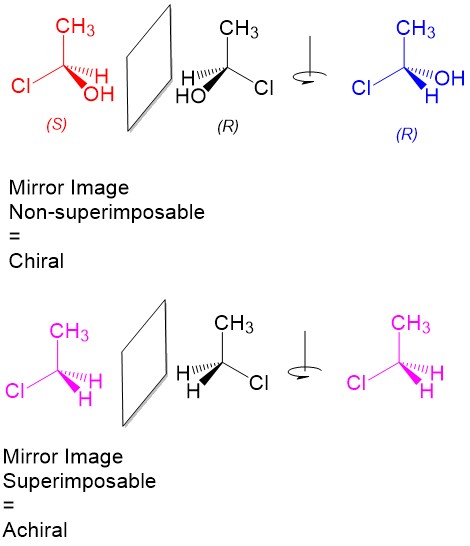
- Stereoisomers – molecules with the same geometrical arrangement (i.e. same connectivity) that are not superimposable on each other. For a carbon center (referred to as a stereocenter), this requires bonding to four different substituents!
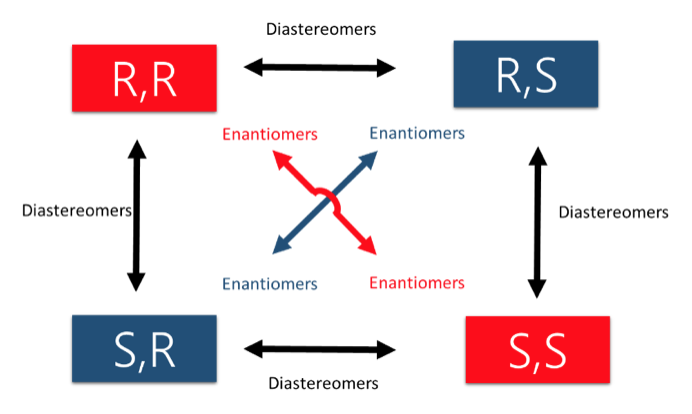
- Enantiomers – A pair of stereoisomers that are mirror images of each other.
- Diastereomers – Any pairing of stereoisomers that are NOT mirror images of each other.
- Meso compounds – A molecule with stereocenters that shows symmetry in reflection. Because of this symmetry, the molecule is considered achiral!
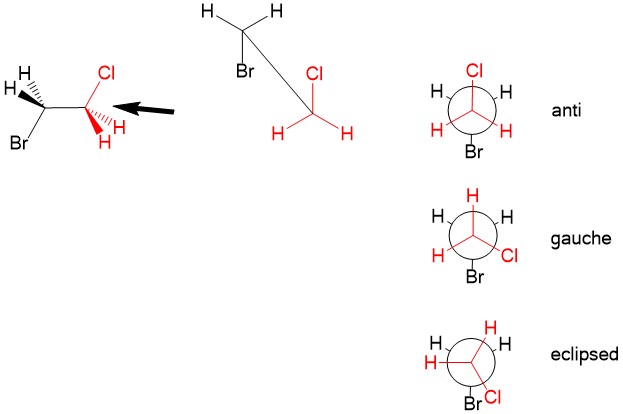
Newman Projections – A way to visualize different rotational conformations of substituent comparing two carbon atoms that looks down their C-C bond, thus showing the alignment of the substituents.
- Gauche conformation – when the angle between two substituents is 60°
- Anti conformation – when the angle between two substituents is 180°
- Eclipsed conformation – when two substituents overlap, or the angle between them is 0°
Stereoselectivity Reaction Terms – describes the reaction conditions that leads to UNEQUAL stereoisomer formation
- Syn addition – addition of substituents on the same face of the place of symmetry across a double bond.
- Anti addition – addition of substituents on the opposite face of the plane of symmetry across a double bond.
- Inversion of configuration – substitution of a nucleophile with the opposite stereochemistry as the starting material (e.g., R ® S). This stereospecificty happens for ALL concerted mechanisms (SN2, E2, etc.)
- Racemization (racemic mixture) – substitution of a nucleophile which produces both stereoisomers (e.g., R ® R + S). This stereospecificity happens for ALL carbocation (SN1, E1, etc.) intermediates.
Regioselectivity Terms – describes the reaction conditions that leads to UNEQUAL constitutional isomer formation.
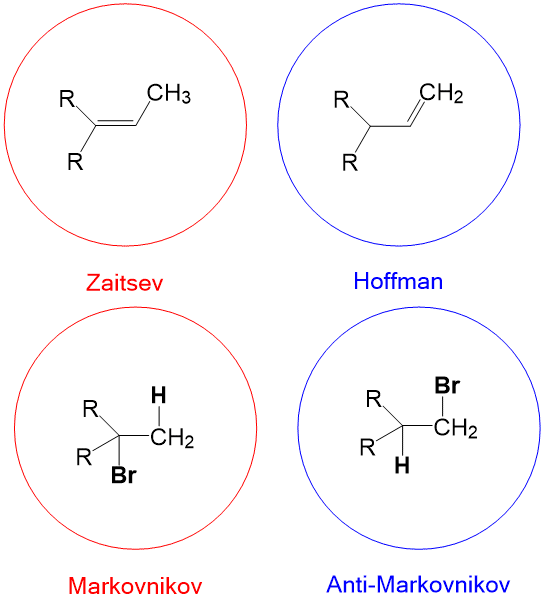
- Markovnikov selectivity – in alkene addition reactions, the placement of hydrogen will occur on the LEAST substituted carbon (a.k.a. carbon center greatest number of hydrogens).
- Anti-Markovnikov selectivity – in alkene addition reactions, the placement of hydrogen will occur on the MOST substituted carbon (a.k.a. carbon center with fewest number of hydrogens).
- Zaitsev product – in elimination reactions, the formation of the alkene with the MOST substituents is favored. (e.g., E2 elimination with a non-bulky base such as sodium ethoxide).
- Hoffman product – in elimination reactions, the formation of the alkene with the LEAST substituents is favored. (e.g., E2 elimination with a bulky base, sodium tert-butoxide).
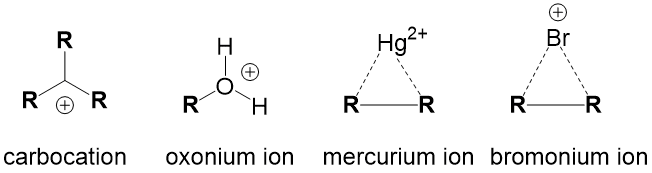
Intermediate Terms – describes intermediates that are key to reaction progression.
- Carbocation – formed any time a leaving group breaks a bond with carbon to generate a carbon center with 3 bonds and a positive charge.
- Oxonium ion – formed any time an alcohol is protonated.
- Mercurium ion – formed in oxymercuration/demercuration addition reactions
- Bromonium ion – formed in halogenation addition reactions of alkenes