It is important to know the hierarchy of Reaction Intermediates such as Radicals, Carbocations, Carbanions.
Here we present a quick guide to Reaction Intermediate hierarchies.
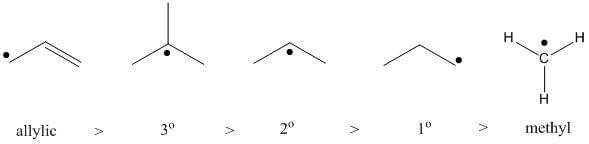
The Big Picture: Radicals and Carbocations prefer a greater degree of alkyl substitution. While, Carbanions are the opposite: Carbanions prefer a lesser degree of alkyl substitution. However, all three prefer the allylic position the most!
This is some of the information presented in Part 10 of our Study Guide: one of the many resources available to StudyOrgo.com members. Learn more about it here: How It Works.
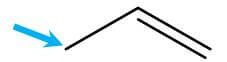
- Allylic position
- Definition: The position immediately next to a double bond
- Image: The arrow points to the allylic position:
- Reaction Intermediates
- Radical
- Typically electrons come in pairs. However there are unpaired electrons known as radical electrons. These are usually just called radicals.
- Radical stability
- Radicals prefer a greater degree of alkyl substitution. Even more so, radicals prefer to be in the allylic position.
- Therefore here is the hierarchy of radical intermediate stability:
- Radical
- Carbocation
- Carbocations serve as electrophiles in reactions. They will attract electrons easily as the carbon is deficient in electrons.
- Carbocation stability
- Carbocations prefer a greater degree of alkyl substitution. Even more so, carbocations prefer to be in the allylic position. Therefore here is the hierarchy of carbocation intermediate stability:
- Carbanion
- Carbanions serve as nucleophiles in reactions. They will donate electrons easily as the carbon has excess electrons.
- Carbanion stability
- Carbanions prefer a lesser degree of alkyl substitution. Even more so, carbanions prefer to be in the allylic position. Therefore here is the hierarchy of carbanion intermediate stability:
Join StudyOrgo.com today and save 10% using coupon code “acespring”. We have the highest pass rate of any organic chemistry study program and we guarantee you pass.