Here is a look at a video tutorial that we include with the reactions to help you learn them backwards and forwards.

Here is a look at a video tutorial that we include with the reactions to help you learn them backwards and forwards.
Epoxides are useful functional groups in organic chemistry for generating reactive centers. Many drugs, both beneficial and harmful, rely on the process of epoxidation to become biologically active. In this article, we will review some of the concepts of epoxidation and give you a preview of the hundreds of reactions explained with clear depictions when you sign up for a membership with StudyOrgo!
There are two processes, ring closing- (epoxidation) and ring opening- reactions. Epoxides contain an oxirane, which is a 3 membered ring that contains an oxygen atom. Preparation of epoxides require a double bond across which the oxygen will be added across the C-C bond to form the oxirane ring.
Ring-Closing Reactions:
Formation of an oxirane ring can be accomplished in 3 ways starting with an alkene reactant. The use of the following peroxides is a common way to prepare an epoxide.
***Memorize both of these reagents– if you ever see them- think epoxides!
Ring-Opening Reactions:
Reaction of epoxoides with any strong nucleophile leads to ring opening and formation of an alcohol via an inter-molecular SN2 reaction. Nucelophiles such as –OH, –OR, –SH, Grignard Reagents and LAH will all attack the epoxide at the least sterically hindered position to break the ring. A practical example of ring opening reactions is the use of ethylene oxide to sterilize medical equipment. Microbes present on the surface of the equipment are exposed to ethylene oxide whereby DNA, RNA and proteins contain many -NH2 and -OH groups to serve as nucleophiles that will react with the epoxide. The result is an alkylated group, which will interfere with cell function and induce cell death, known as apoptosis.
The second example explains the organic chemistry of the widely-used monomer Bis-Phenol-A, which has drawn attention for its potentially carcinogenic properties, is reacted with the epoxide, epichlorohydrin, to form polymers used in many plastic products. Note that upon ring-opening of the epoxide in Step 1, a halohydrin is instantly formed and can is further reacted in Step 2 with NaoH in a ring-closing reaction to regenerate the epoxide for another round of catalysis in Step 3, so a long strand of the BPA polymer is formed.
Sign up with StudoOrgo today to get more explanations and clear-cut examples of the mechanisms required in your Organic Chemistry 1 & 2 course today!
One reaction that always troubles students taking organic chemistry is the Diels-Alder reaction. In this article, we will discuss the basics of the reaction and give you a preview of the clear-cut examples of organic chemistry reaction mechanisms available to StudyOrgo members!
The Reaction:
The Diels-Alder reaction is referred to as a pericyclic reaction, in that two reactants cyclize to become one ring. The reaction utilizes a [4+2] cycloaddition electron mechanism; meaning that the first reactant has 4 pi-electrons and the second reactant has 2 pi-electrons. These are named the diene (2 alkenes, or 4 pi-electrons) and the dieneophile (1 alkene, or 2 pi-electrons, that “seeks” the diene). The diene MUST be in the cis- conformation in order to cyclize, the trans- isoform would not form the ring… try it with your models or on paper!
The Mechanism:
Below is the reaction mechanism using arrow-pushing. This mechanism occurs in a concerted, or one-step, process. It is thought that the dienophile attacks the diene and rearranges the electron distribution to form the two new red C-C bonds and results in a new 2 pi-electron bond. Energetically, breaking 3 pi-bonds in the reactants and formation of the 2 sigma-bonds + 1 pi-bond in the product has a negative enthalpy value (deltaH), therefore the reaction is exothermic and spontaneous!
Stereoselectivity:
Most examples students encounter are more complex, where the dieneophile has substituents and the diene is already cyclized, thus forming a bridged product. Depending on the orientation of the dienophile with respect to the diene, two products are possible. Below is an example of such a reaction.
In this example, only one product is observed; the endo- product. The selectivity for this reaction can be illustrated in the following diagram. The addition of the reaction occurs via the “left-handed rule” that is, if you put your left hand thumb along the dieneophile and twist to the left (see red arrows), the rotation of the dienophile represents the stereochemistry of the substituents after the reaction is complete. Two products are possible, the exo- and endo- products. The reason endo- is preferred can be seen by looking at the Neumann Projection along the bolded bond. In the exo- product, the Cl group is gauche to the Bridge group. In the endo- product, the CL group is anti to the Bridge group. Thus, steric hindrance is a major factor in determining the stereochemistry and since all reaction will deal with these steric factors, endo- will always be preferred!
Sign up today at StudyOrgo for more details and examples to improve your organic chemistry grade!
Deciphering 1H-NMR Spectra
One of the most important concepts taught in organic chemistry is the method for determining the chemical structure of newly synthesized or unknown compounds. In this article, we will summarize the concept of proton NMR, the most common NMR information acquired by organic chemists.
While proton NMR is used every day in the real world by organic chemists, it is also tested every day in the real world by organic chemistry professors. That is why we are bringing you this exclusive StudyOrgo.com simplified review on NMR.
Before you read on, if you like what you see, but are looking for explanations on different organic chemistry topics and reactions, check out how our signature online organic chemistry program works to help you earn higher grades.
Now if you are ready to read on about NMR- here ya go:
Placing an unknown sample in a strong magnetic field allow 1H nuclei (99.98% abundance) to “resonate”, which is when their nuclear spins flip at a unique electromagnetic (EM) frequency (Hertz, Hz). The instrument detects this and plots it on a graph in units of ppm. This value is relative to an internal standard, tetramethylsilane (TMS) which is arbitrarily set at 0 ppm. The signals originating from your unknown sample are recorded as a net difference from the TMS reference. The ranges for common functional groups are shown in the graph below.
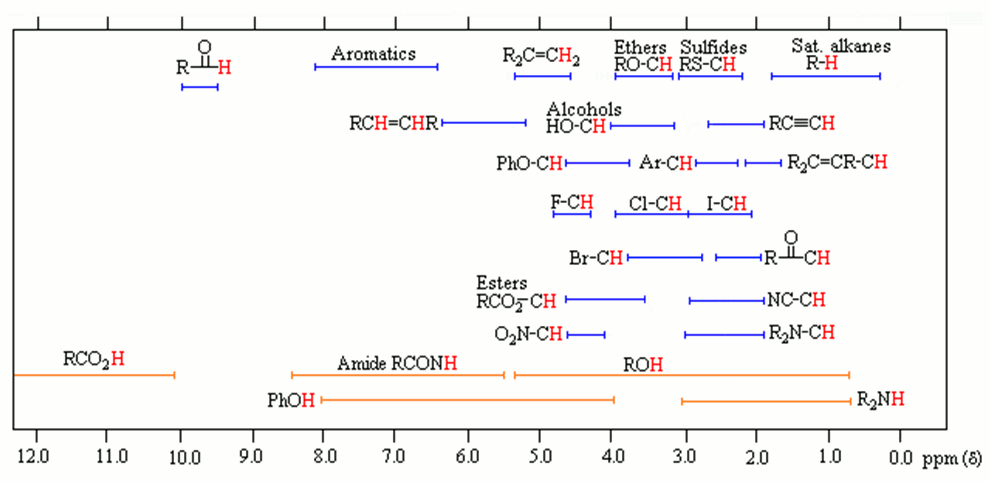
Notice that protons (in red) neighboring or attached to electronegative elements (N,O,S,Cl,Br,I) have a large chemical shift (d). This is colloquially referred to as “downfield” on the spectrograph. This is due to the deshielding effect, which is simply that the electron cloud around protons near electronegative or electron-withdrawing groups are smaller, so less EM radiation is required to resonate the nucleus. Conversely, when the proton is connected to carbons, the chemical shift is <2 ppm. This is because these proton nuclei are “shielded” from the magnetic field and higher energy is required for them to resonate and referred to as “upfield” on the spectrograph. Once a NMR spectrograph is recorded, 4 pieces of information can be determined from the data as long as the chemical formula of the compound is known.
To illustrate the points, we will consider the following 1H-NMR spectrum of the C5H10O.
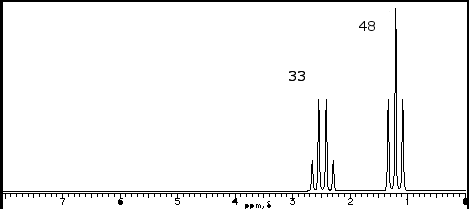
This is the easiest to interpret. The number of peaks correspond to the number of unique, or chemically indistinguishable, hydrogen nuclei. There are two peaks on the graph, therefore of the 10 hydrogens in the molecule, there are two types.
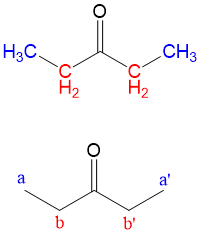
The two peaks on the spectrum are located at (d)2.42 and (d)1.07. Remembering that the chemical formula includes 5 carbons and one oxygen, it is clear there is a good deal of symmetry to the molecule. Furthermore, the only proton near an oxygen that exhibits a chemical shift ~ 2.5 with is neighboring a carbonyl group HCRC=O. So far, we know our compound is symmetric and has a carbonyl group, therefore only 4 carbons can have carbons.
Integration is the calculation of the area beneath the peaks. This information is determined by the computer software and would not be required for you to determine from visual inspection. But if it is provided, it will instantly determine the number of “equivalent” (or chemically indistinguishable) hydrogens in your graph. Looking at our spectra, we have integration of 33 + 48 = 81 cart units in total. The integration fraction can be multiplied by the total number of hydrogens from the chemical formula to determine number of equivalent hydrogens. For instance, at (d)2.42 [33/81 = 0.40 * 10 total hydrogens = 4 hydrogens at (d)2.42]. Therefore, we know that the protons near the carbonyl total 4. Given the symmetry from the low peak count, we know these hydrogens must be two CH2 groups. The same for the peak at (d)1.07 [48/81 = 0.60 * 10 total hydrogens = hydrogens at (d)1.07]. Since only 2 carbons are left, we must conclude these are terminal CH3 groups.
Signal splitting occurs from a phenomenon of coupling, which occurs when NON-equivalent neighboring protons interfere with the resonance of a proton nuclei. The degree of splitting occurs in an N+1 rule. For instance, at d1.07 we have a triplet. This indicates that there are 2 protons neighboring the CH3 group that are different. This would have to be the protons from the CH2 group at (d)2.42. Similarly, the peak at (d)2.42 as a quartet, indicating that 3 protons are neighboring the CH2 group that are different. This would have to be the protons from the CH3 group.
And voila! The chemical structure from the spectrum must be 3-propanone. This is an example of the clear-cut explanations you will receive with your membership with StudyOrgo. With practice and help from StudyOrgo, you’ll be solving your organic reaction problems in no time!
Want to try it about before you purchase? No problem- check out our sample reaction flashcard page.
Ready to sign up? Sign up here.

Use your iPad or iPhone to study on the go during your break.
One of the questions we receive here at StudyOrgo frequently is how to get ahead for the next semester. Arguably, the second semester of organic chemistry is much more challenging for students than the first. This is because all of the general chemistry refresher and organic chemistry introductory material has been covered and you are now responsible for it! You may have covered addition reactions, maybe substitution and elimination if your professor was on task. But that covers the reactivity of only 3 of the more than 20 functional groups you will study in this class! Thus, the speed of the course is about to pick up. We have developed a few helpful hints to take advantage of your winter break and get ready for the spring semester of organic chemistry.
With a little time management and StudyOrgo, you will have no trouble getting an A in Organic Chemistry this year!