As Orgo 2 lectures start up, there will be a number of concepts that will be covered quickly to speed up the class so you can learn lots of functional group transformations this semester. In this article, we will discuss the concepts and theories of Thermodynamics. While the topic sounds complicated, we at StudyOrgo have extensive experience instructing principles and reaction mechanisms frequently covered in Organic Chemistry. Sign up today for clear, detailed explanations of over 180 Orgo Chem reactions and reviews on conceptual topics!
Aromatic compounds contain conjugated systems of pi bonds, meaning one pi bond after another, that are arranged in a ring. This creates a current of electron density that circles the compound. If the pi electrons fill the bonding molecular orbitals, the compound becomes aromatic! But if too many pi electrons are present, the non-bonding and antibonding orbitals become occupied, and the compound is now anti-aromatic.
Aromatic compounds are UNUSUALLY STABLE and have important chemical and synthetic uses. How do we know if the compound is aromatic or not?
Here is a short list of rules lists the properties of aromatic compounds.
The Huckel aromaticity rules are:
- Molecule is cyclic
- Have one pi orbital per atom of the ring
- Planar, in an SP2 hybridized orbital, over every atom of the ring
- Have a closed loop of 4n+2 pi-bond electrons, where n is equal to any integer (0,1,2,3,…)
However, anti-aromatic compounds have an unusual INSTABILITY to them.
The Huckel anti-aromaticity rules are:
- Molecule is cyclic
- Have one p orbial per atom of the ring
- Be planar, in an sp2 hybridized orbital, over every atom of the ring
- But, anti-aromatic compounds have a closed loop of 4n pi-bond electrons.
Many times students are asked to describe the molecular orbitals of aromatic compound examples. How do we know what they look like? An easy way to describe the molecular orbitals of small conjugated rings is to draw a Frost Circle. To do this, follow the steps below.
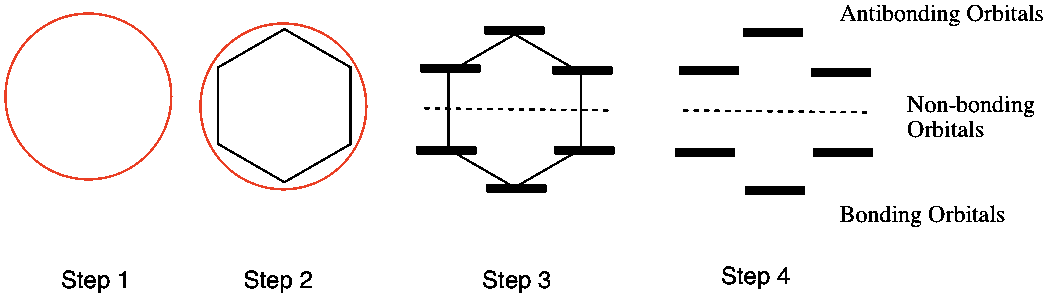
- Draw a circle
- Connect the carbon atoms to the circle such that one is always pointed at the bottom
- Draw a horizontal line at each carbon – this represents the pi bond molecular orbitals
- Draw a dotted line through the middle of the circle – this is the boundary between bonding and antibonding orbitals.
- Anything pi electrons occupying an orbital below the line are in a bonding orbital and are STABLE
- Any pi electrons on the line or above the line are in non-bonding & anti-bonding orbital and are UNSTABLE.
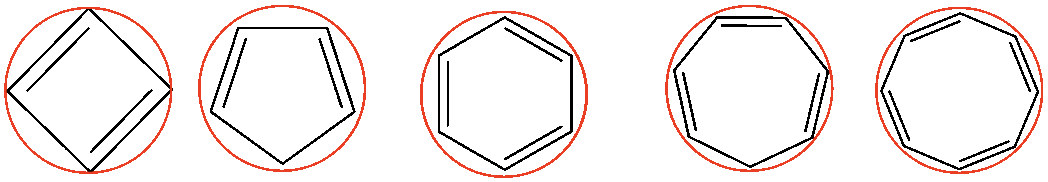
Below are the Frost Circles for many types of rings.
Before you begin, make sure the Huckel rules apply; do you have enough electrons? Is it SP2 hybridized? Is it a ring? Is it planar? Some aromatic rings are heterocyclic (elements other than carbon) and ionic (meaning one of the elements in the ring has a charge). If you have the Huckel rules applied, then determine the number of pi electrons and fill in the Frost Circle.
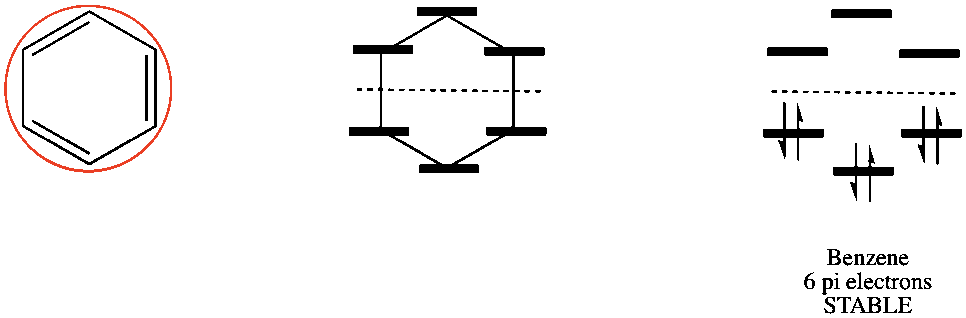
Here is butane.
Three double bonds = 6 pi electrons. Paired together, all of the pi electrons are in bonding orbitals so benzene is in fact aromatic.
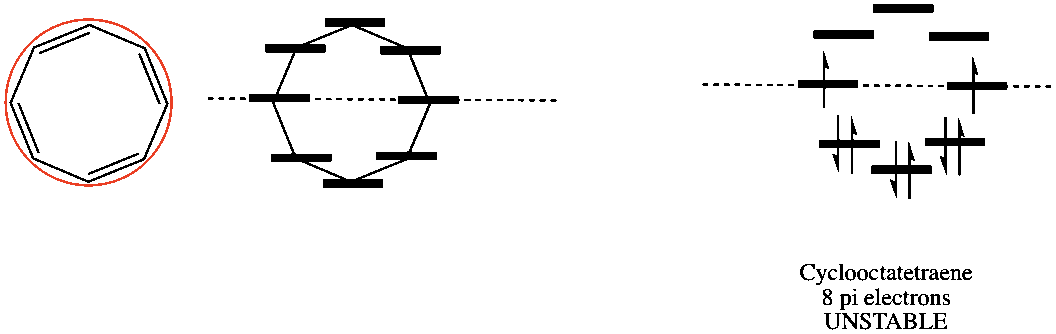
Here is cyclooctatetraene.
Four double bonds = 8 pi elecrons, Pairing the first 6 electrons in the bonding orbitals, we place last 2 electrons in each of the non-bonding orbitals. Because of these two electrons, this molecule is anti-aromatic!
Practice, practice, practice! This is our suggestion to learn these tricks. As you advance to aromatic reactions, it will be much easier to find shortcuts to faster synthesis schemes and solve more complicated examples which will surely be on your final exam. Sign up with StudyOrgo.com today to master over 180 organic chemistry reactions and learn the mechanisms fast!