This is the fourth and final part of a multi-part module on Free Radical Halogenation.
View the first part here: Part 1: The Mechanism
View the second part here: Part 2: Regioselectivity
View the third part here: Part 3: Stereoselectivity
Question 1:
What principle accounts for the observed regioselectivity of radical bromination that is not observed for radical chlorination of alkenes?
- Lechatlier’s Principle
- Avagadro’s Lab
- Hammond’s Postulate
- Markovnikov Rule
Question 2:
Which product can NOT be prepared in high yield by radical halogenation of alkanes?
- Iodoethane
- 2-Bromo-2-methylheptane
- Chlorocyclopentane
- 2-Bromo-2,4,4,trimethylpentane
Question 3:
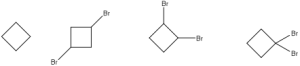
Predict the major product of the following reaction.
Question 4:
Describe the reaction conditions to produce the following product
- NaBr
- Br2
- H2SO4, Br2
- HBr
Answers and Explanations:
Question 1: 3
Difficulty Level: Easy
Explanation: Hammond’s Postulate describes the stability of the radical center is outweighed by the extreme exothermicity of radical chlorination (in contrast to bromination, which is endothermic), thus a mixture of chlorinated products is observed.
Question 2: 1
Difficulty Level: Medium
Explanation: Answers 2,3,4 all would start from alkanes containing secondary and tertiary carbon centers, which produce stable radical intermediates for halogenation. Ethane contains two primary carbons coupled to the extreme endothermicity of iodination would yield very little product.
Question 3: B
Difficulty Level: Medium
Explanation: The steric strain of the cyclopropane ring will drive the hemolytic cleavage of the 2,4 sigma bond with radical bromine to produce a radical intermediate on carbon 2. Propagation of the radical bromine will result in the formation of product B. Product C and D are not possible while the reagents to produce product A are not listed.
Question 4: 4
Difficulty Level: Hard
Explanation: Addition across the double bond with one equivalent of Br would more easily take place via an electrophilic addition using an equivalent of HBr, while radical bromination would produce dibromo-pentane.
There are multiple examples of this reaction to review in the StudyOrgo.com Study Mode. When ready, test your knowledge in the StudyOrgo.com Quiz Mode.
Not a member yet? Sign-Up today!