This is the first part of a multi-part module on Free Radical Halogenation.
There are THREE critical steps to free radical reactions – Memorize!
1) Initiation: The Br2 single bond is broken by high energy ligh (hv) to form radicals placing one electron on each atom.
2) Propagation: (Hint: One radical reacts with a sigle bond to form another radical, thus propigating the radical species to drive the reaction forward.
a) Radical Br abstracts one hydrogen from a C-H bond in propane to form radical propane and HBr.
b) Radical propane asbracts one Br from Br2 to form the bromoalkane and radical Br, thus restoring the reactants for another round as shown in step 2a.
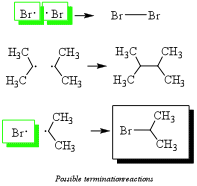
3) Termination: Any two radicals combine to form a single bond. These species will be in low abundance. Hint: Radicals are destroyed by combining two radicals to form a single bond. This eliminates the radical necessary for radical alkane formation (green boxes) as shown in step 2a and ends the reaction.
There are multiple examples of this reaction to review in the StudyOrgo.com Study Mode. When ready, test your knowledge in the StudyOrgo.com Quiz Mode.
Not a member yet? Sign-Up today!